Fine chemicals
产品信息:
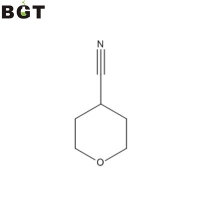
What is 4-Cyanotetrahydropyran? 4-Cyanotetrahydropyran is a valuable element as a starting material or a man-made intermediate for medicine, farming chemical substances, etc, developed for making nitrile compound, carboxylic acidity compound or carboxylate compound. Utilization of 4-Cyanotetrahydropyran 4-Cyanotetrahydropyran can be utilized to synthetize 4-aminomethyltetrahydropyran and its acidity salt 4-aminomethyltetrahydropyran hydrochloride. 4-aminomethyltetrahydropyran (CAS NO. 130290-79-8) and 4-aminomethyltetrahydropyran hydrochloride (CAS No. 389621-78-7) are valuable compounds that could be starting materials or man-made intermediates of medicines, farming chemical substances, and so on. Synthesis of 4-aminomethyltetrahydropyran Within an autoclave made of stainless outfitted having a stirring device, a thermometer along with a pressure gauge and getting an inner volume of 200 ml were filled 10 g (90. mmol) of four-cyanotetrahydropyran, 50 g of twenty-two Percent by weight ammonia-methanol solution and 2 g (17. mmol when it comes to a nickel atom) of developed Raney nickel, and also the mixture was responded under hydrogen atmosphere (.51 to .61MPa) at 45 to 55°C for 17 hrs under stirring. After completing the response, insoluble materials were strained, and also the filtrated material was cleaned with 30 ml of methanol. The filtrate and also the cleaned solution were combined and concentrated under reduced pressure, after which, the concentrate was distilled under reduced pressure (73 to 74°C, 2.67 kPa) to provide 7.94 g (Isolation yield 76.6%) of 4-aminomethyltetrahydropyran in the form of color liquid. The current invention further pertains to a procedure for planning a 4-aminomethyltetrahydropyran as well as an acidity salt thereof from the 4-cyanotetrahydropyran. The nitrile compound, the carboxylic acidity compound or even the carboxylic acidity ester compound, some-replaced tetrahydropyran (4-cyanotetrahydropyran, tetrahydropyran-4-carboxylic acidity) and also the 4-aminomethyltetrahydropyran as well as an acidity salt thereof are helpful compounds as a beginning material or a synthetic intermediate of medications, farming chemicals, etc. As a technique for preparing 4-aminomethyltetrahydropyran as well as the acidity salt from 4-cyanotetrahydropyran, there has been known of a technique, for instance, by which 4-cyanotetrahydropyran and hydrogen are responded in the existence of Raney nickel in anhydrous ethanol (for instance, see Worldwide Patent Publication No. 94/05639). However, based on this process, yield from the objective product, 4-aminomethyltetrahydropyran, is low, and a lot of a by-product (bis(4-tetrahydropyranylmethyl)amine) is created, etc., to ensure that this is not satisfying as an industrial process for preparing 4-aminomethyltetrahydropyran as well as its acidity salt. Synthesis of 4-aminomethyltetrahydropyran hydrochloride To some reaction vessel made of glass outfitted having a stirring tool and a thermometer and an inner volume of 3 L were filled with the previously-stated concentrate and 833 ml of tetraethylenepentamine, the mixture was stirred at 105 to 115°C for two hrs. After completing the stirring, the stated solution was distilled under reduced pressure (70 to 80°C, 1.73 to 4.67 kPa) to acquire 1430.2 g 4-aminomethyltetrahydropyran from the distilled solution .
For a process to prepare 4-acyltetrahydropyran, there has been revealed a method that, for instance, 2,2'-dichloroethyl ether and cyanoethyl acetate are responded to synthesize ethyl 4-cyanotetrahydropyran-4-carboxylate. The resulting compound is hydrolyzed to organize 4-cyanotetrahydropyran-4-carboxylic acidity, then, the acidity is heated under high temperature to synthesize 4-cyanotetrahydropyran (Approaches for creating alkyl 3-(4-tetrahydropyranyl)-3-oxopropionate compound and 4-acyltetrahydropyran) Ways to synthetize 4-cyanotetrahydropyran After completing the response, the response mixture was cooled to 0°C, and 1760 g (22. mol) of the 50% sodium hydroxide aqueous solution was progressively added dropwise. After completing the dropwise addition, the mixture was exposed to hydrolysis at 70°C for one hour. After completing the response, the response mixture was cooled to 0°C, and a pair of.21 L (26.5 mol) of 35% muriatic acidity was progressively added dropwise thereto. After completing the dropwise addition, the mixture was stirred at 70°C for half an hour.
联系我
与我的联系人共享
4-Cyanotetrahydropyran MSDS.pdf
Download
供应单标签
|