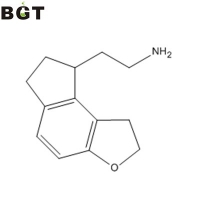
What is (s)-2-(1 ,6,7,8-tetrahydro-2h-indeno[5,4-b]furan-8-yl)ethylamine?
(s)-2-(1,6,7,8-tetrahydro-2h-indeno[5,4-b]furan-8-yl)ethylamine is a key intermediate for synthetizing Ramelteon, a MT1 and MT2 melatonin receptor selective agonist.
Brief introduction of ramelteon
Previously, sleep agents bind to GABAA receptors which are associated with anxiolytic, myorelaxant, and amnesic effects, such as with drugs like zolpidem, eszopiclone, and zaleplon. As the first in a new class of sleep agents, Ramelteon selectively binds to the MT1 and MT2 receptors in the suprachiasmatic nucleus (SCN). It was developed by Takeda Pharmaceuticals North America with the trade name Rozerem, and was approved by the U.S. Food and Drug Administration (FDA) on July 4th, 2005 for long-term use. It can be used for insomnia, delayed sleep onset in particular. There is no proof showing that Ramelteon produces dependence and any potential for abuse. What's more, while there is withdrawal and rebound insomnia typically with GABA modulators, it is not shown in ramelteon. Ramelteon can also be used for the treatment of Delayed sleep phase syndrome. Ramelteon was proven by practice to be associated with a lower risk of delirium (3% vs 32%; P = .003). According to Chakraborti, D; Tampi, D. J.; Tampi, R. R. (2014) ( "Melatonin and Melatonin Agonist for Delirium in the Elderly Patients". American journal of Alzheimer's disease and other dementias), "ramelteon was found to be beneficial in preventing delirium in medically ill individuals when compared to placebo."
The way to synthetize (s)-2-(1 ,6,7,8-tetrahydro-2h-indeno[5,4-b]furan-8-yl)ethylamine
Step 1: With (±)-2-(1,6,7,8-Tetrahydro-2H-indeno[5,4-B]fur-8-yl)ethanamin the raceme as raw material, resolve it with Di-p-anisoyl-L-tartaric acid the optically pure organic acid in the resolution solvent and get the organic acid salt. The aforesaid solvent can be one or more of methanol, ethanol, tetrahydrofuran, and acetonitrile. Among them, methanol, ethanol, and acetonitrile are preferred.
Step 2: Recrystalize the organic acid salt gained from step 1 in the solvent and get the optically pure (s)-2-(1 ,6,7,8-tetrahydro-2h-indeno[5,4-b]furan-8-yl)ethylamine organic acid salt crystal.
Step 3: Dissolve the product from step 2 in the NaOH solvent and extract (s)-2-(1 ,6,7,8-tetrahydro-2h-indeno[5,4-b]furan-8-yl)ethylamine from it with methylbenzene or dichloromethane.
This synthesis process reduces the production cost and is easy to operate. It avoids the purification process after the reaction, and thus is feasible for scale-up production.